null
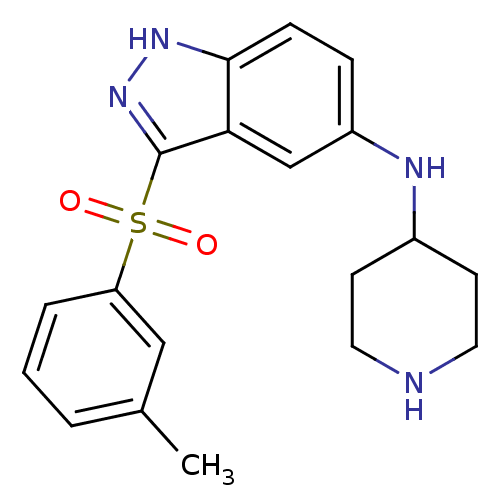
SMILES Cc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12
InChI Key InChIKey=JLKHMSUYQUHJJS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50334754
Found 2 hits for monomerid = 50334754
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Antagonist activity at cloned human 5-HT6 receptor expressed in human HeLa cells assessed as inhibition of 5HT-induced cyclic AMP formationMore data for this Ligand-Target Pair