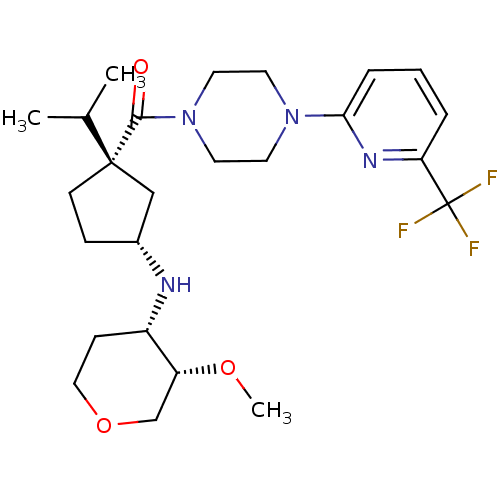
null
SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cccc(n1)C(F)(F)F
InChI Key InChIKey=MMFPYPNUFANCKT-ZSTWQFLISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50337618
Found 7 hits for monomerid = 50337618
Affinity DataIC50: 6.20nMAssay Description:Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma countingMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Displacement of MCP-Alexa 488 from CCR2 in human whole blood after 5 mins by flow cytometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 18.8nMAssay Description:Antagonist activity at human CCR2 by whole blood assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Displacement of labeled dofetilide from human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 27.5nMAssay Description:Displacement of [125I]MCP1 from human CCR2 preincubated for 30 mins by human whole cell binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Displacement of labeled MIP-1beta from human CCR5 receptorMore data for this Ligand-Target Pair