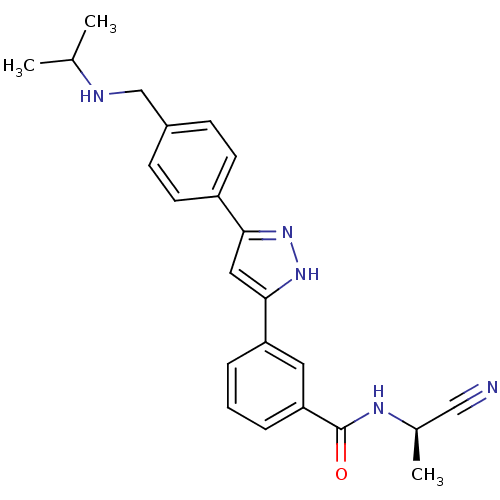
null
SMILES CC(C)NCc1ccc(cc1)-c1cc([nH]n1)-c1cccc(c1)C(=O)N[C@H](C)C#N
InChI Key InChIKey=TXDVLJPWDLIFHD-MRXNPFEDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50337697
Found 2 hits for monomerid = 50337697
TargetSerine/threonine-protein kinase D1(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human PKD1 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 530nMAssay Description:Inhibition of PKD1 in rat neonatal ventricular myocytes expressing GFP-HDAC5 assessed as inhibition of phosphorylation-dependent HDAC5 nuclear exportMore data for this Ligand-Target Pair