null
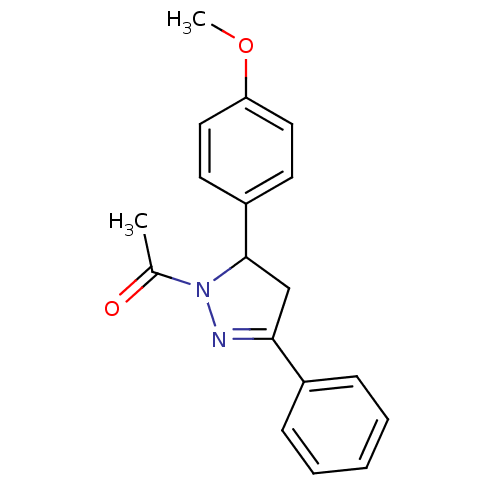
SMILES COc1ccc(cc1)C1CC(=NN1C(C)=O)c1ccccc1
InChI Key InChIKey=ZPDSCMQRJLPXDN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50338855
Found 3 hits for monomerid = 50338855
Affinity DataKi: 1.41E+5nMAssay Description:Non-competitive inhibition of goat liver cathepsin H using Leu-betaNA substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.49E+5nMAssay Description:Non-competitive inhibition of goat liver cathepsin B using BANA substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Bos taurus (Bovine))
Indo-Soviet Friendship College of Pharmacy
Curated by ChEMBL
Indo-Soviet Friendship College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 7.62E+4nMAssay Description:Inhibition of bovine Xanthine oxidase assessed as decrease in uric acid production preincubated at 293 nM of compound for 5 mins by spectrophotometryMore data for this Ligand-Target Pair