null
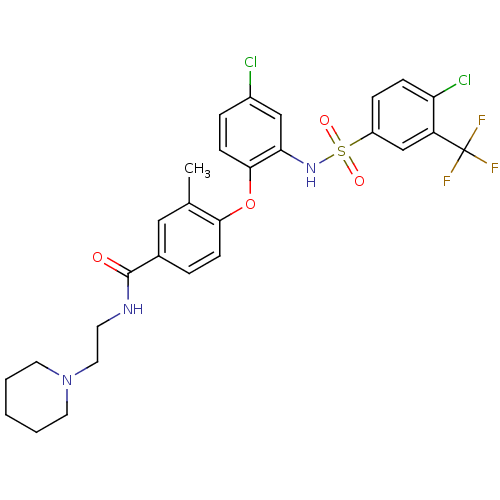
SMILES Cc1cc(ccc1Oc1ccc(Cl)cc1NS(=O)(=O)c1ccc(Cl)c(c1)C(F)(F)F)C(=O)NCCN1CCCCC1
InChI Key InChIKey=QFGIJXOCXFOLFD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50359020
Found 2 hits for monomerid = 50359020
Affinity DataKi: 25nMAssay Description:Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrsMore data for this Ligand-Target Pair
Affinity DataKd: 2.51E+3nMAssay Description:Antagonist activity at CCR2 receptor in human whole blood assessed as inhibition of MCP-1 induced monocyte shape change pretreated for 15 mins before...More data for this Ligand-Target Pair