null
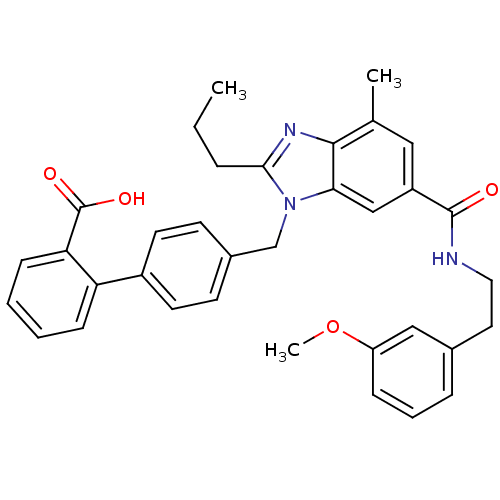
SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cccc(OC)c1
InChI Key InChIKey=NCAWYFSYLMNGPT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50363830
Found 2 hits for monomerid = 50363830
TargetType-2 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma countingMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 30.8nMAssay Description:Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma countingMore data for this Ligand-Target Pair