null
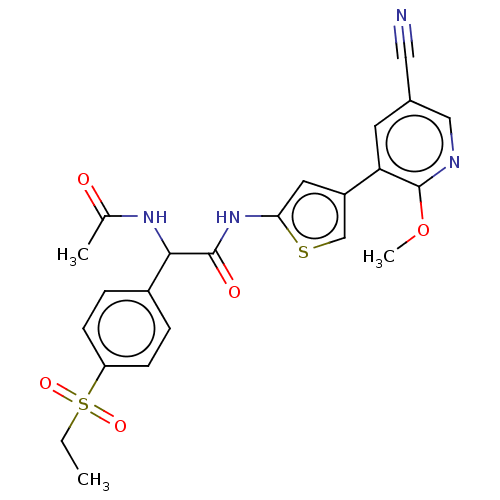
SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(C)=O)C(=O)Nc1cc(cs1)-c1cc(cnc1OC)C#N
InChI Key InChIKey=HUJRJJNOPIQEAC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50369034
Found 3 hits for monomerid = 50369034
Affinity DataIC50: 67nMAssay Description:Inverse agonist activity at biotinylated HN-Avi-MBP-TCS-human RORgammat (258 to 518 residues) assessed as inhibition of biotinylated SRC-1 peptide NC...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Displacement of [3H]-2-(4-(ethylsulfonyl)phenyl)-N-(4-(2-(methoxymethyl)phenyl)thiophen-2-yl)acetamide from purified N-(HN)6-GST-TCS-human RORgammat ...More data for this Ligand-Target Pair
Affinity DataIC50: 990nMAssay Description:Inverse agonist activity at RORgammat in human TH17 cells assessed as inhibition of IL17 release incubated for 4 days by HTRF assayMore data for this Ligand-Target Pair