null
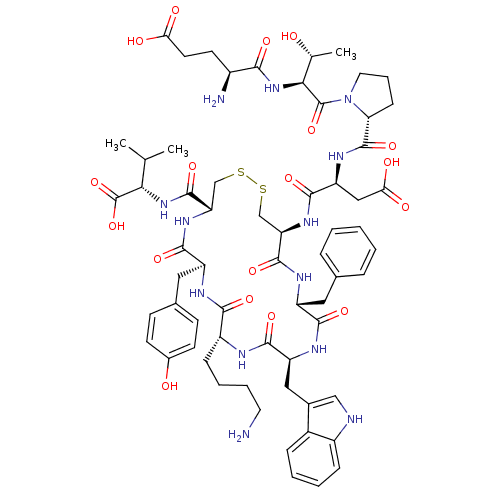
SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O
InChI Key InChIKey=HFNHAPQMXICKCF-FDMLFMOBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50378580
Found 9 hits for monomerid = 50378580
Affinity DataKi: 0.794nMAssay Description:Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.794nMAssay Description:Binding affinity towards human Urotensin 2 receptor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 0.794nMAssay Description:Ability to displace radioligand [125I]Tyr-hU-II from human recombinant Urotensin 2 receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 5.01nMAssay Description:Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contractionMore data for this Ligand-Target Pair
Affinity DataEC50: 0.832nMAssay Description:Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstrictionMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 0.832nMAssay Description:Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstrictionMore data for this Ligand-Target Pair
Affinity DataEC50: 1.09nMAssay Description:Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair