null
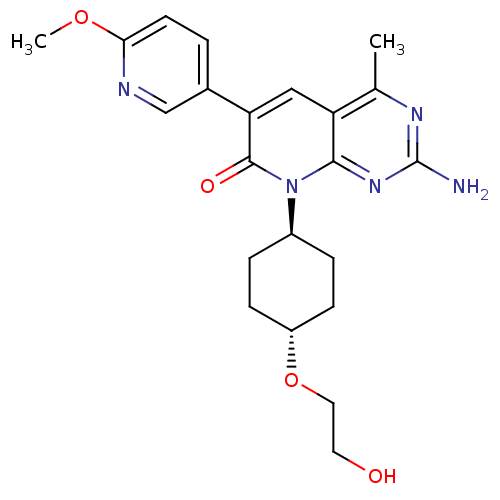
SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O
InChI Key InChIKey=XDLYKKIQACFMJG-WKILWMFISA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 24 hits for monomerid = 50380313
Found 24 hits for monomerid = 50380313
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Mus musculus (Mouse))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Inhibition of mouse PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))TBA
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))TBA
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))TBA
Affinity DataKi: 16nMAssay Description:Inhibition of mTOR (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Mus musculus (Mouse))TBA
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atriaMore data for this Ligand-Target Pair
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Mus musculus (Mouse))TBA
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atriaMore data for this Ligand-Target Pair
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Mus musculus (Mouse))TBA
Affinity DataIC50: 1.90nMAssay Description:Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atriaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of choline acetyltransferase (ChAT) activityMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))TBA
Affinity DataIC50: 2.10nMAssay Description:Inhibition of choline acetyltransferase (ChAT) activityMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))TBA
Affinity DataIC50: 1.60nMAssay Description:Inhibition of choline acetyltransferase (ChAT) activityMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))TBA
Affinity DataIC50: 1.90nMAssay Description:Inhibition of squalene synthetase was determined in rat liver microsomesMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Mus musculus (Mouse))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atriaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of AKT phosphorylation at Ser 473 in human BT20 cellsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate compound preincubated for 15 mins by luciferase-based luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of mTOR (unknown origin) after 40 mins by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of mTOR (unknown origin) assessed as inhibition of 4EBP-1 phosphorylation preincubated for 15 mins before substrate addition by TR-FRET as...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of squalene synthetase was determined in rat liver microsomesMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 4.85nMpH: 7.4 T: 2°CAssay Description:Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate preincubated for 15 mins before substrate addition by luciferase-based luminescen...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)