null
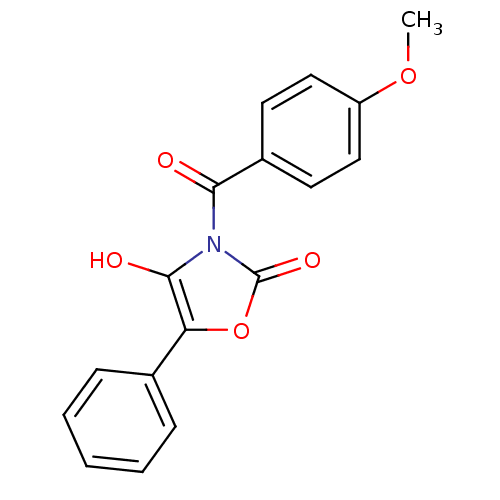
SMILES COc1ccc(cc1)C(=O)n1c(O)c(oc1=O)-c1ccccc1
InChI Key InChIKey=NJSUMZHNMUHUQM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50384104
Found 3 hits for monomerid = 50384104
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of cathepsin G using Suc-Ala-Ala-Pro-Phe-p-nitroanilide as substrate after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of human neutrophil elastase using chromogenic substrate (N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide after 120 minsMore data for this Ligand-Target Pair