null
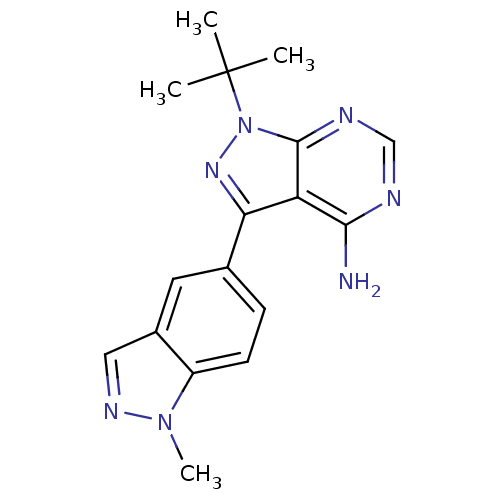
SMILES Cn1ncc2cc(ccc12)-c1nn(c2ncnc(N)c12)C(C)(C)C
InChI Key InChIKey=NLPBHIMGPVMLNX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50389736
Found 3 hits for monomerid = 50389736
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human SRC using Ac-EIYGEFKKK-OH as substrate after 60 mins by phosphorimaging methodMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human tyrosine kinases.More data for this Ligand-Target Pair