null
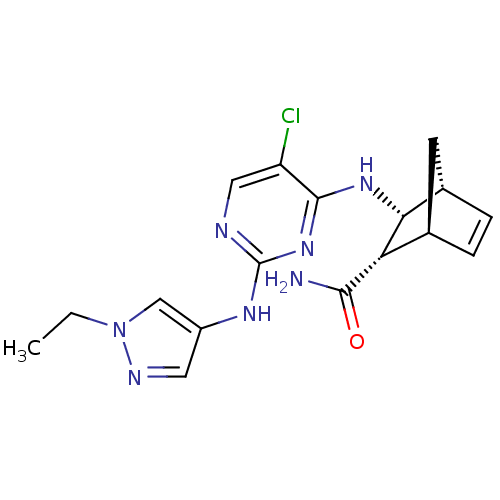
SMILES CCn1cc(Nc2ncc(Cl)c(N[C@@H]3[C@@H]4C[C@@H](C=C4)[C@@H]3C(N)=O)n2)cn1
InChI Key InChIKey=WBVVEHDODCFLBV-XXSPCDMZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50389988
Found 3 hits for monomerid = 50389988
Affinity DataIC50: 8nMAssay Description:Inhibition of Aurora B kinase by HTRF analysis in presence of 1 mM ATPMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of KDR by HTRF analysis in presence of 1 mM ATPMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human KDR autophosphorylation expressed in mouse NIH/3T3 cellsMore data for this Ligand-Target Pair