null
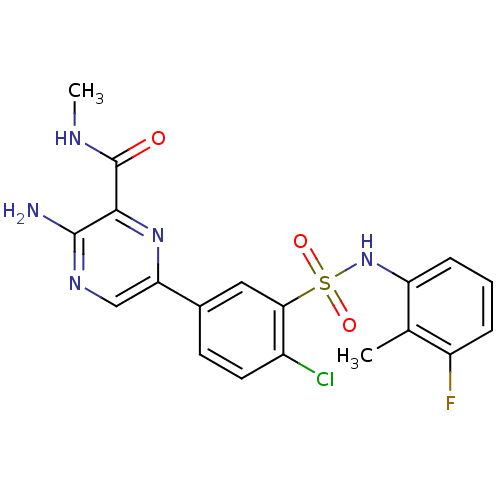
SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1C
InChI Key InChIKey=LFOKRWIYRIYGNF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 50393329
Found 23 hits for monomerid = 50393329
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.15E+3nMAssay Description:Inhibition of human PI3Kbeta expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescenc...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataIC50: 415nMAssay Description:Inhibition of human PI3Kdelta expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescen...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of AKT1More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of c-MetMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of CDC7More data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.81E+3nMAssay Description:Inhibition of CRAF1More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of FGFR1More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of Flt3More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of IRKMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of JAK2More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of KDRMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of Platelet-derived growth factor subunit BMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of PDK1More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of SRCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+3nMAssay Description:Inhibition of mTORMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 805nMAssay Description:Inhibition of human PI3Kgamma expressed in C5a-stimulated mouse RAW 264.7 cells assessed as inhibition of AKT phosphorylation by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of Aurora kinase BMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of CHK1More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of CDK1/CyclinBMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 342nMAssay Description:Inhibition of human PI3Kalpha expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescen...More data for this Ligand-Target Pair