null
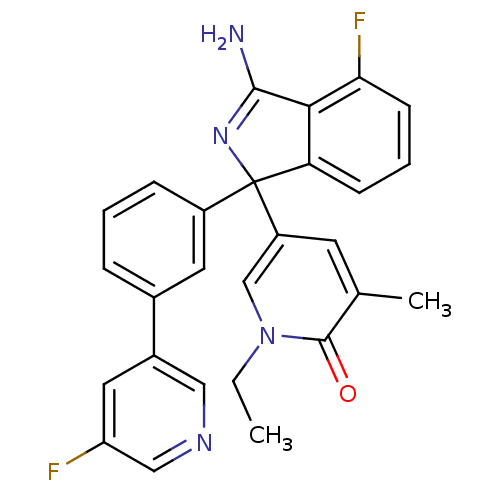
SMILES CCn1cc(cc(C)c1=O)C1(N=C(N)c2c1cccc2F)c1cccc(c1)-c1cncc(F)c1
InChI Key InChIKey=FLNASPMZRNYEQE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50398269
Found 3 hits for monomerid = 50398269
Affinity DataIC50: 4nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by IonWorks assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair