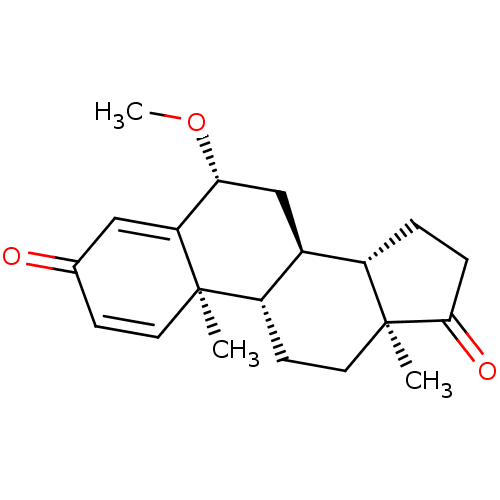
null
SMILES CO[C@@H]1C[C@H]2[C@@H]3CCC(=O)[C@@]3(C)CC[C@@H]2[C@@]2(C)C=CC(=O)C=C12
InChI Key InChIKey=DMKRZIKUGNDODB-XOJMLDNQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50398455
Found 1 hit for monomerid = 50398455
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair