null
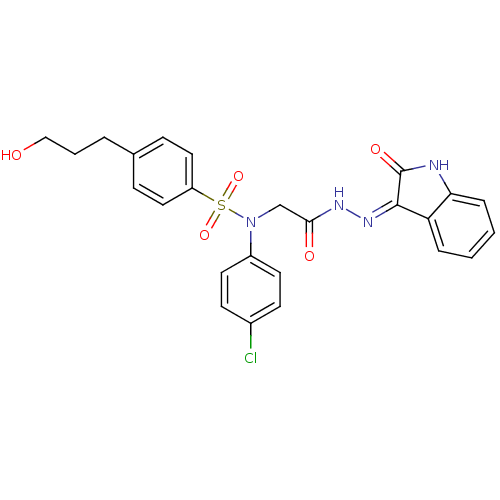
SMILES OCCCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1
InChI Key InChIKey=VMLXQVRAOYTIFJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50410623
Found 4 hits for monomerid = 50410623
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibitory activity against human Oxytocin induced intracellular Calcium mobilization in human Oxytocin receptor transfected HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against cytochrome P450 2C19 isoformMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 345nMAssay Description:Inhibitory activity against cytochrome P450 2C9 isoformMore data for this Ligand-Target Pair