null
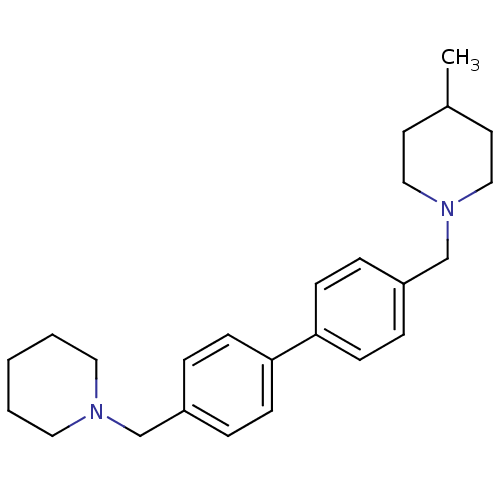
SMILES CC1CCN(Cc2ccc(cc2)-c2ccc(CN3CCCCC3)cc2)CC1
InChI Key InChIKey=DTQQVJLJZAQLBK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50412464
Found 3 hits for monomerid = 50412464
Affinity DataKi: 2.40nMAssay Description:Displacement of [3H]RAMHA from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Rattus norvegicus (rat))
Universit£ degli Studi di Parma
Curated by ChEMBL
Universit£ degli Studi di Parma
Curated by ChEMBL
Affinity DataKi: 5.75nMAssay Description:Displacement of [3H]RAMHA from histamine H3 receptor in Wistar rat brain membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+3nMAssay Description:Inhibition of acetylcholinesterase in Wistar rat brain homogenate by Ellman's methodMore data for this Ligand-Target Pair