null
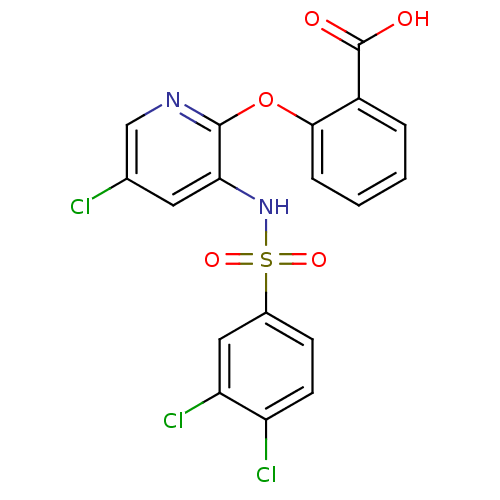
SMILES OC(=O)c1ccccc1Oc1ncc(Cl)cc1NS(=O)(=O)c1ccc(Cl)c(Cl)c1
InChI Key InChIKey=GQLXQNMFKWOOSD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50416285
Found 9 hits for monomerid = 50416285
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataKi: 1.26nMAssay Description:Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 4(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Antagonist activity at CCR4More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 251nMAssay Description:Antagonist activity at CCR5More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 3.98E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 2.14E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 3.16E+4nMAssay Description:Binding affinity to human ERG by fluorescence-polarization binding assayMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 3.98E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair