null
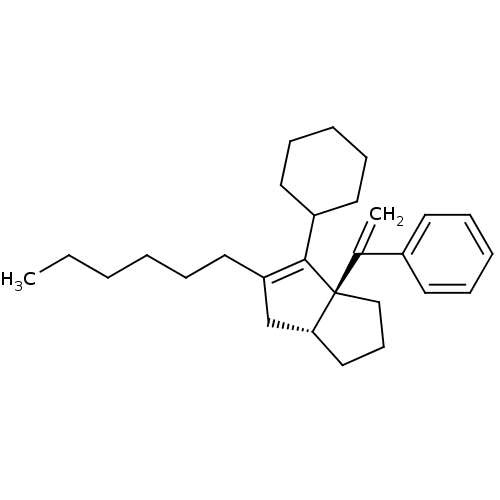
SMILES CCCCCCC1=C(C2CCCCC2)[C@@]2(CCC[C@@H]2C1)C(=C)c1ccccc1
InChI Key InChIKey=LLUBAJDYDAQGSK-IAPPQJPRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50418317
Found 2 hits for monomerid = 50418317
TargetNuclear receptor subfamily 5 group A member 2(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataEC50: 1.26E+3nMAssay Description:Agonist activity at human LRH-1 receptor assessed as TIF2 737-757 peptide recruitment by TR-FRET assay relative to controlMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Agonist activity at human SF-1 assessed as DAX1 1-23 peptide recruitment by TR-FRET assay relative to controlMore data for this Ligand-Target Pair