null
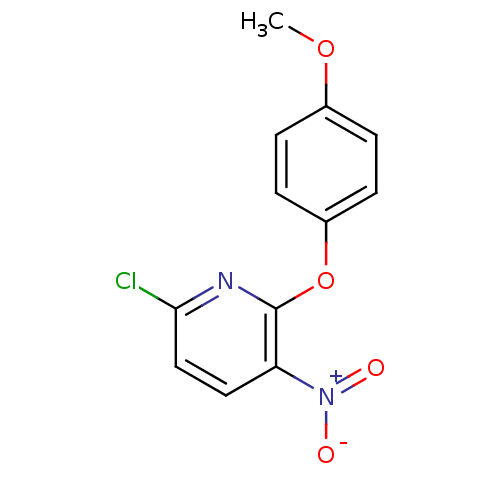
SMILES COc1ccc(Oc2nc(Cl)ccc2[N+]([O-])=O)cc1
InChI Key InChIKey=IPZJMCGPCSSZJS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50421322
Found 3 hits for monomerid = 50421322
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair