null
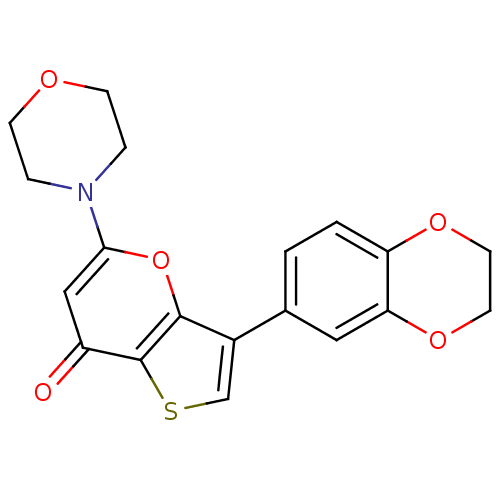
SMILES O=c1cc(oc2c(csc12)-c1ccc2OCCOc2c1)N1CCOCC1
InChI Key InChIKey=BYTKNUOMWLJVNQ-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 22 hits for monomerid = 50427453
Found 22 hits for monomerid = 50427453
TargetBromodomain-containing protein 4 [75-147](Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 241nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 34nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 214nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 960nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 158nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase pim-1(Homo sapiens (Human))
The University of Arizona
Curated by ChEMBL
The University of Arizona
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of PIM1 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
The University of Arizona
Curated by ChEMBL
The University of Arizona
Curated by ChEMBL
Affinity DataIC50: 289nMAssay Description:Inhibition of mTOR (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1.31E+3nMAssay Description:Inhibition of PI3K p110gamma (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 34nMAssay Description:Inhibition of PI3K p110alpha (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 960nMAssay Description:Inhibition of PI3K p110delta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 214nMAssay Description:Inhibition of PI3K p110beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 241nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
Affinity DataIC50: 214nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 960nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 158nMAssay Description:Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
The University of Arizona
Curated by ChEMBL
The University of Arizona
Curated by ChEMBL
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
SignalRx Pharmaceuticals, Inc.
US Patent
SignalRx Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1.55E+3nMAssay Description:Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)