null
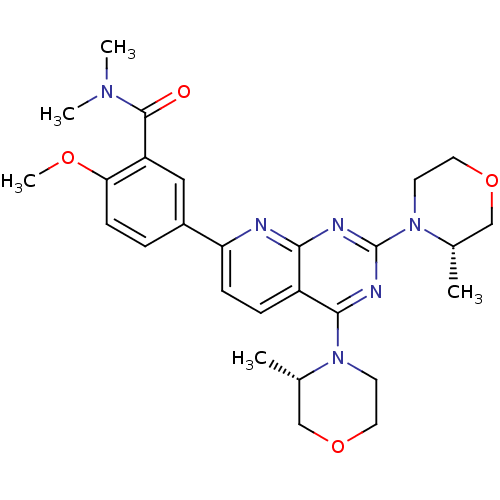
SMILES COc1ccc(cc1C(=O)N(C)C)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C
InChI Key InChIKey=RCFKHMOMPCIKFL-ROUUACIJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50429704
Found 2 hits for monomerid = 50429704
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
KUDOS PHARMACEUTICALS LIMITED
US Patent
KUDOS PHARMACEUTICALS LIMITED
US Patent
Affinity DataIC50: 42nMT: 2°CAssay Description:The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi...More data for this Ligand-Target Pair