null
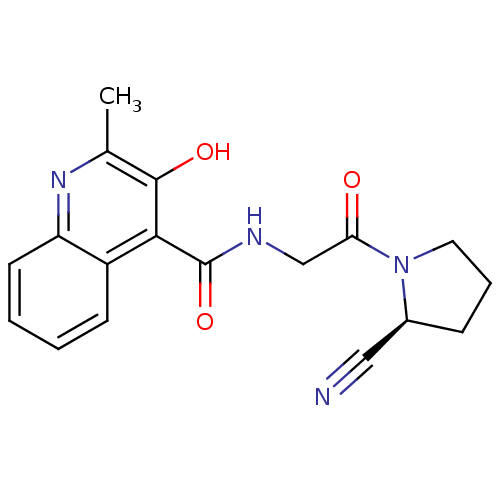
SMILES Cc1nc2ccccc2c(C(=O)NCC(=O)N2CCC[C@H]2C#N)c1O
InChI Key InChIKey=QPYHHKIZAVGBJJ-LBPRGKRZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50434175
Found 7 hits for monomerid = 50434175
TargetProlyl endopeptidase FAP(Mus musculus (Mouse))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 5.90E+3nMpH: 8.3 T: 2°CAssay Description:Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 1.00E+5nMpH: 8.3 T: 2°CAssay Description:Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 9(Homo sapiens (Human))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 1.00E+5nMpH: 8.3 T: 2°CAssay Description:Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ...More data for this Ligand-Target Pair
TargetProlyl endopeptidase FAP(Mus musculus (Mouse))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi...More data for this Ligand-Target Pair
TargetProlyl endopeptidase(Homo sapiens (Human))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 5.36E+4nMpH: 8.3 T: 2°CAssay Description:Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ...More data for this Ligand-Target Pair
TargetProlyl endopeptidase(Homo sapiens (Human))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 5.36E+4nMAssay Description:Inhibition of human recombinant PREP expressed in Escherichia coli using Z-Gly-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substra...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 2(Homo sapiens (Human))
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
Affinity DataIC50: 1.00E+5nMpH: 8.3 T: 2°CAssay Description:Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ...More data for this Ligand-Target Pair