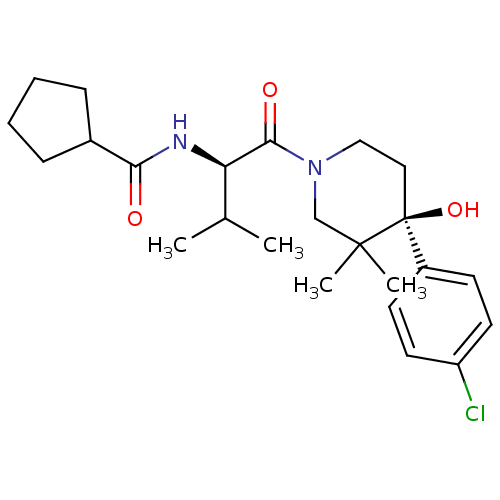
null
SMILES CC(C)[C@@H](NC(=O)C1CCCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1
InChI Key InChIKey=UTGYFIZUYRNNRM-YKSBVNFPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50436284
Found 3 hits for monomerid = 50436284
Affinity DataKi: 0.700nM ΔG°: -12.5kcal/moleT: 2°CAssay Description:For radioligand competition studies, a final concentration of 1x105 THP-1 monocytic leukemia cells are combined with 100 μg of LS WGA PS beads (...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Binding affinity to human CCR1More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxisMore data for this Ligand-Target Pair