null
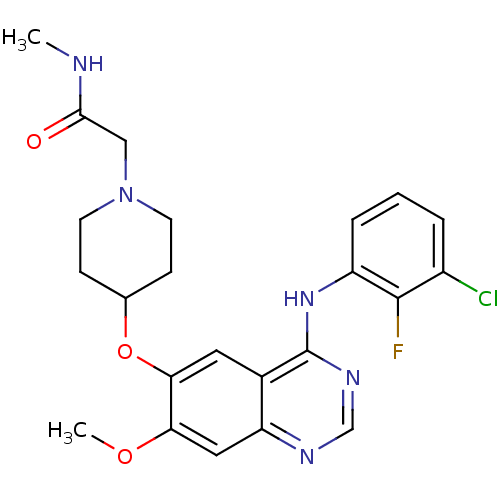
SMILES CNC(=O)CN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC
InChI Key InChIKey=DFJSJLGUIXFDJP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50437353
Found 10 hits for monomerid = 50437353
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human EFGR expressed in baculovirus/Sf21 system by ELISAMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Tsinghua University
Curated by ChEMBL
Tsinghua University
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human HER2 expressed in baculovirus/Sf21 system in presence of ATP by ELISAMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of EGFR in human A431 cells assessed as reduction in EGF-stimulated EGFR autophosphorylation preincuabted for 90 mins followed by EGF-stim...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Tsinghua University
Curated by ChEMBL
Tsinghua University
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human HER2 expressed in baculovirus/Sf21 system by ELISAMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-3(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human HER3 expressed in baculovirus/Sf21 system by ELISAMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of human EGFR expressed in baculovirus/Sf21 system in presence of ATP by ELISAMore data for this Ligand-Target Pair