null
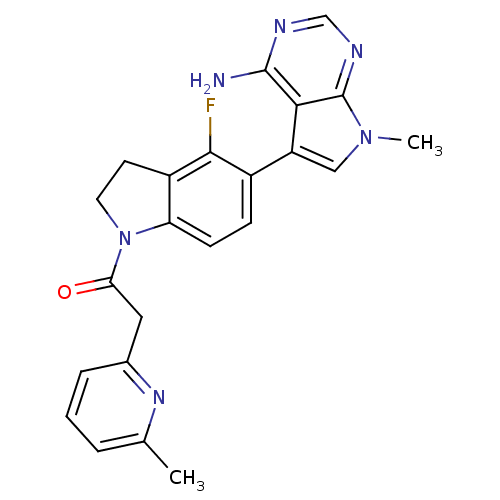
SMILES Cc1cccc(CC(=O)N2CCc3c2ccc(-c2cn(C)c4ncnc(N)c24)c3F)n1
InChI Key InChIKey=PRWSIEBRGXYXAJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50442166
Found 11 hits for monomerid = 50442166
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of thapsigargin-induced autophosphorylation of PERK in human A549 cells preincubated for 1 hr followed by thapsigargin-induction measured ...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 3(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of GST-tagged PERK cytoplasmic domain (536 to 1116) (unknown origin) assessed as biotinylated His6-tagged EIF2alpha phosphorylation preinc...More data for this Ligand-Target Pair
TargeteIF-2-alpha kinase GCN2(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.39E+3nMAssay Description:Inhibition of GCN2 (unknown origin) assessed as EIF2AK4 phosphorylationMore data for this Ligand-Target Pair
TargetInterferon-induced, double-stranded RNA-activated protein kinase(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 905nMAssay Description:Inhibition of PKR (unknown origin) assessed as EIF2AK2 phosphorylationMore data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 2-alpha kinase 1(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of HRI (unknown origin) assessed as EIF2AK1 phosphorylationMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 60 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 5 to 60 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.47E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 60 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 60 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair