null
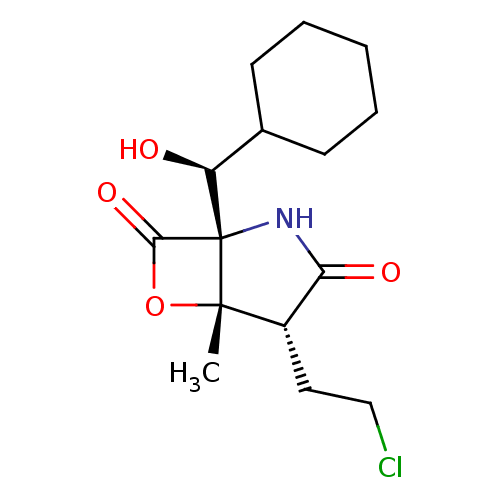
SMILES C[C@@]12OC(=O)[C@@]1(NC(=O)[C@@H]2CCCl)[C@@H](O)C1CCCCC1
InChI Key InChIKey=OJZFVOGTYOBPKD-GVARAGBVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50444464
Found 2 hits for monomerid = 50444464
TargetProteasome subunit beta type-5(Homo sapiens (Human))
The Pennsylvania State University
Curated by ChEMBL
The Pennsylvania State University
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of chymotrypsin like activity of human 20S proteasome after 1 hr by luminescence assayMore data for this Ligand-Target Pair
TargetProteasome subunit beta type-5(Homo sapiens (Human))
The Pennsylvania State University
Curated by ChEMBL
The Pennsylvania State University
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Inhibition of chymotrypsin like activity of 20S proteasome in HEK293 cells after 24 hrs by microscopic analysisMore data for this Ligand-Target Pair