null
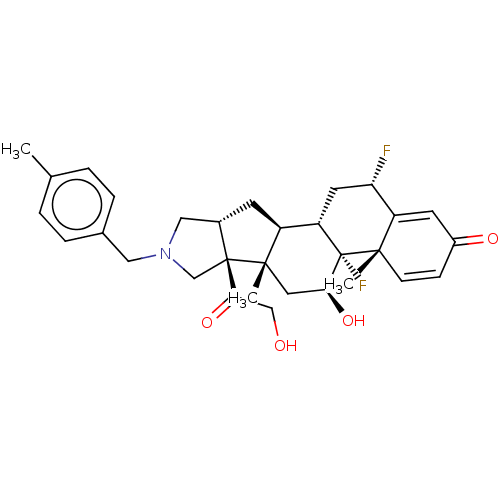
SMILES [H][C@]12CN(Cc3ccc(C)cc3)C[C@@]1(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]([H])(C[C@H](F)C4=CC(=O)C=C[C@]34C)[C@]1([H])C2
InChI Key InChIKey=UUHWUZKGTKYLGH-MIWRREBKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50456007
Found 2 hits for monomerid = 50456007
Affinity DataKi: 0.520nMAssay Description:Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in insect cells after 24 hrs by scintillation proximity as...More data for this Ligand-Target Pair
Affinity DataEC50: 8.40nMAssay Description:Induction of nuclear translocation of human recombinant ProLabel-tagged glucocorticoid receptor expressed in CHO-K1 cells after 3 hrs by luminescence...More data for this Ligand-Target Pair