null
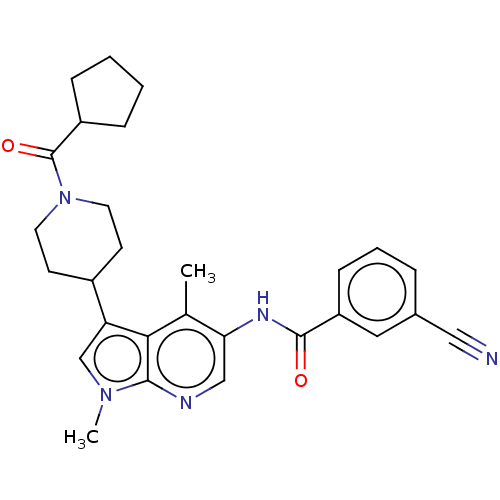
SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CCCC3)c12
InChI Key InChIKey=RMNDQPLEKNTPNK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50466911
Found 2 hits for monomerid = 50466911
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISAMore data for this Ligand-Target Pair