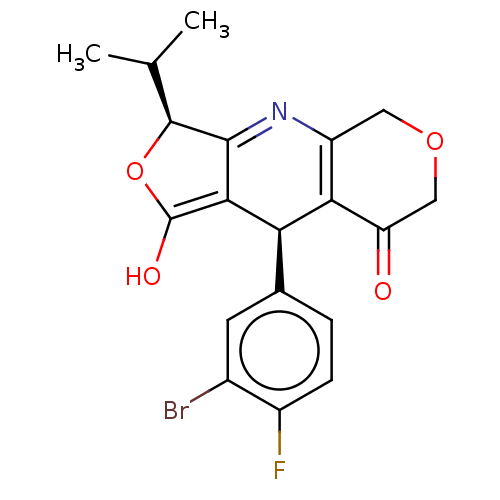
null
SMILES CC(C)[C@@H]1OC(O)=C2[C@@H](C3=C(COCC3=O)N=C12)c1ccc(F)c(Br)c1
InChI Key InChIKey=SGEYYGCVCUZKNF-KDOFPFPSSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50475957
Found 2 hits for monomerid = 50475957
TargetATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 1.20E+3nMAssay Description:Activity against pig bladder KATP channel opening assessed as ability to relax field-stimulated pig detrusorMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: <1.00E+4nMAssay Description:Ability to open human urinary bladder Kir6.2 channel containing SUR2B in Ltk cells by FLIPR assayMore data for this Ligand-Target Pair