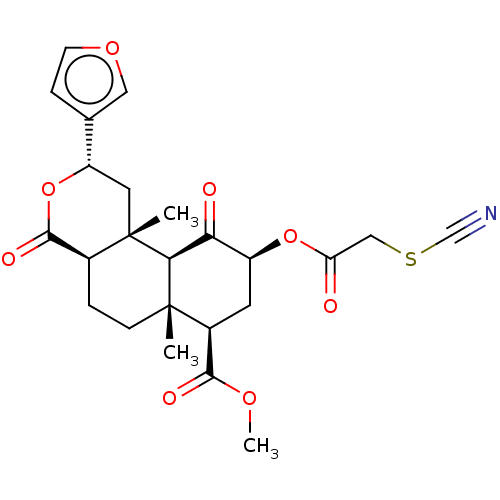
null
SMILES [H][C@@]12CC[C@@]3(C)[C@@H](C[C@H](OC(=O)CSC#N)C(=O)[C@]3([H])[C@@]1(C)C[C@H](OC2=O)c1ccoc1)C(=O)OC
InChI Key InChIKey=AZPUAKGNQXURGA-ZWLNRFIDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50491417
Found 9 hits for monomerid = 50491417
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DADLE from delta opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 0.590nMAssay Description:Binding affinity to kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.0770nMAssay Description:Inhibition of kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 5.20nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin) expressed in HEK cells co-expressing luciferase based cAMP biosensor assessed as increase ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.13E+3nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin) assessed as increase in venus-tagged N-terminal beta-arrestin-2 recruitment by BRET assayMore data for this Ligand-Target Pair