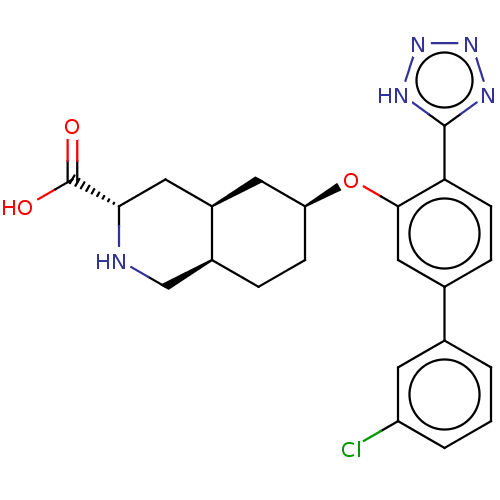
null
SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1cccc(Cl)c1
InChI Key InChIKey=HVPPBDRUBZSELE-OQKRBJEGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50494348
Found 2 hits for monomerid = 50494348
TargetGlutamate receptor ionotropic, kainate 1(Homo sapiens (Human))
Centro de Investigaci�n Lilly
Curated by ChEMBL
Centro de Investigaci�n Lilly
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Displacement of [3H]ATPA from human Gluk1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin)More data for this Ligand-Target Pair