null
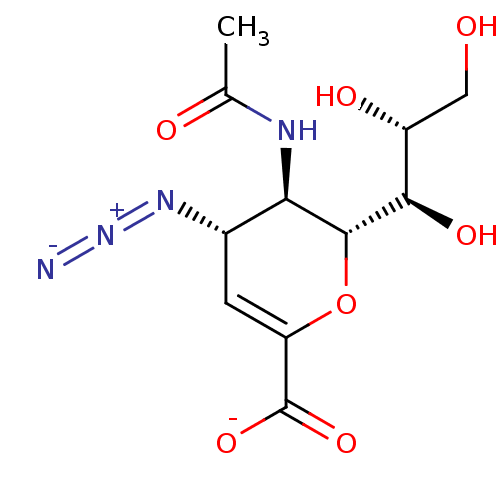
SMILES [Na;v0+].[H][C@]1([#8]-[#6](=[#6]-[#6@H](-[#7]=[N+]=[#7-])-[#6@H]1-[#7]-[#6](-[#6])=O)-[#6](-[#8-])=O)[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8]
InChI Key InChIKey=RNDTUIVDJRSLTC-VCFRRRQNSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50497285
Found 1 hit for monomerid = 50497285
Affinity DataIC50: 3.92E+3nMAssay Description:Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by...More data for this Ligand-Target Pair