null
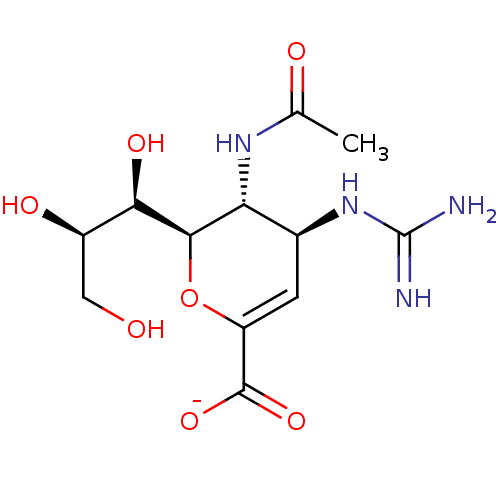
SMILES [Na;v0+].[H][C@]1([#8]-[#6](=[#6]-[#6@H](-[#7]-[#6](-[#7])=[#7])-[#6@H]1-[#7]-[#6](-[#6])=O)-[#6](-[#8-])=O)[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8]
InChI Key InChIKey=MOHYGJPDTFGLMY-VCFRRRQNSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50497287
Found 1 hit for monomerid = 50497287
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by...More data for this Ligand-Target Pair