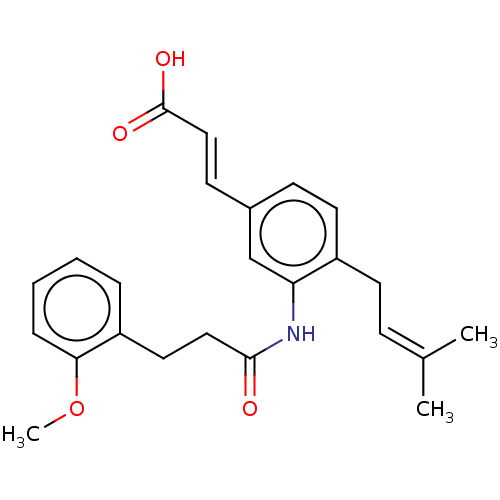
null
SMILES [#6]-[#8]-c1ccccc1-[#6]-[#6]-[#6](=O)-[#7]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#6]\[#6]=[#6](\[#6])-[#6]
InChI Key InChIKey=ITJWNJNADAZNEJ-XNTDXEJSSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50509731
Found 2 hits for monomerid = 50509731
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
Texas Tech University Health Sciences Center
Curated by ChEMBL
Texas Tech University Health Sciences Center
Curated by ChEMBL
Affinity DataIC50: 81nMAssay Description:Inhibition of recombinant human AKR1C3 expressed in Escherichia coli BL21 (D3) using S-tetralol as substrate in presence of NADP+ by UV-spectrophotom...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Texas Tech University Health Sciences Center
Curated by ChEMBL
Texas Tech University Health Sciences Center
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of recombinant human AKR1C2 expressed in Escherichia coli BL21 (D3) using S-tetralol as substrate in presence of NADP+ by UV-spectrophotom...More data for this Ligand-Target Pair