null
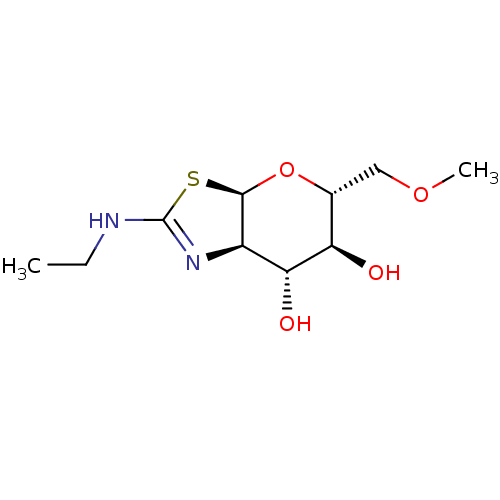
SMILES [H][C@]12O[C@H](COC)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NCC)S2
InChI Key InChIKey=AVBFRSQZSIUFPO-JGKVKWKGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50513936
Found 8 hits for monomerid = 50513936
Affinity DataKi: 190nMAssay Description:Inhibition of recombinant human OGAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Nav1.5 (unknown origin)More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
Merck & Co.
Curated by ChEMBL
Merck & Co.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Cav1.2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck & Co.
Curated by ChEMBL
Merck & Co.
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXR (unknown origin) assessed as CYP3A4 inductionMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck & Co.
Curated by ChEMBL
Merck & Co.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of MK-0499 from human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair