null
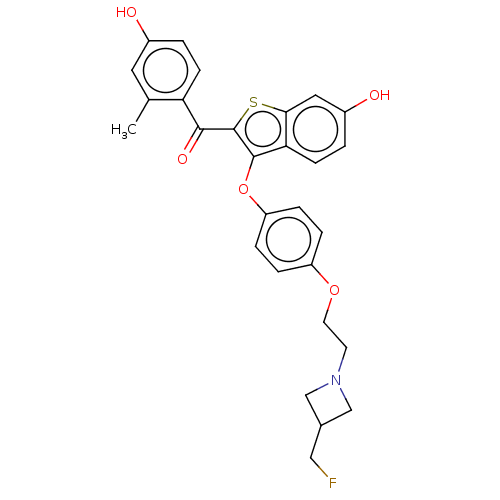
SMILES Cc1cc(O)ccc1C(=O)c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CC(CF)C2)cc1
InChI Key InChIKey=QDABNZLSCGLKNY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50514294
Found 2 hits for monomerid = 50514294
Affinity DataIC50: 5.10nMAssay Description:Inhibition of ERalpha in human MCF7:WS8 cells assessed as increase in degradation of ERalpha incubated for 24 hrs by In-Cell Western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Antagonist activity at ERalpha in human MCF7:WS8 cells incubated for 18 hrs by dual luciferase reporter gene assay relative to controlMore data for this Ligand-Target Pair