null
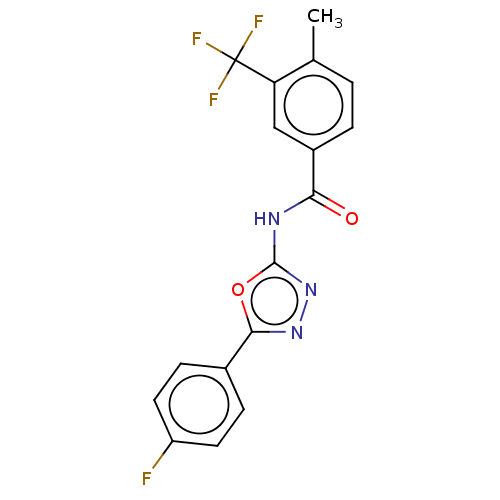
SMILES Cc1ccc(cc1C(F)(F)F)C(=O)Nc1nnc(o1)-c1ccc(F)cc1
InChI Key InChIKey=APOYWYOEJPPGTP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50521103
Found 2 hits for monomerid = 50521103
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of human AC1 expressed in HEK293 cells assessed as decrease in A23187-stimulated cAMP accumulation preincubated for 30 mins followed by A2...More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of human AC8 expressed in HEK293 cells assessed as decrease in A23187-stimulated cAMP accumulation preincubated for 30 mins followed by A2...More data for this Ligand-Target Pair