null
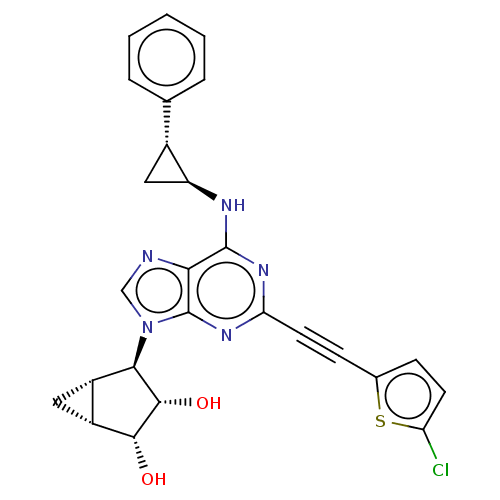
SMILES [H][C@@]12C[C@]1([H])[C@H]([C@H](O)[C@@H]2O)n1cnc2c(N[C@H]3C[C@@H]3c3ccccc3)nc(nc12)C#Cc1ccc(Cl)s1
InChI Key InChIKey=ZJRBBCPVLLPWIQ-FLPYBPNLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50527829
Found 2 hits for monomerid = 50527829
Affinity DataKi: 81nMAssay Description:Displacement of [125I]I-AB-MECA from recombinant human A3AR expressed in HEK293 cell membranes measured after 60 mins by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataKi: 1.14E+3nMAssay Description:Displacement of [125I]I-AB-MECA from recombinant mouse A3AR expressed in HEK293 cell membranes measured after 60 mins by liquid scintillation countin...More data for this Ligand-Target Pair