null
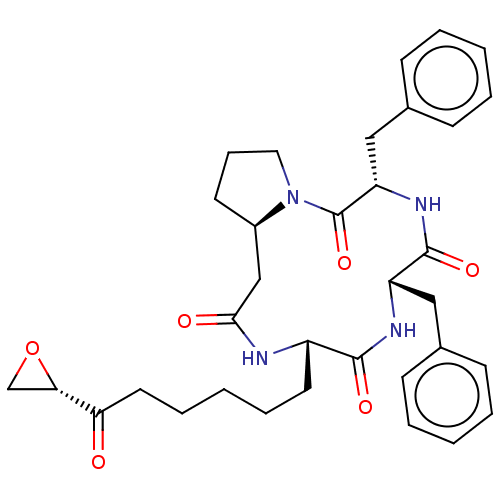
SMILES [H][C@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCC(=O)[C@@H]1CO1)NC(=O)C2
InChI Key InChIKey=TUJYSRGFWBQJNM-QUBFWBSFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50531385
Found 2 hits for monomerid = 50531385
TargetHistone deacetylase 1(Homo sapiens (Human))
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of recombinant human HDAC1 expressed in NIH/3T3 cells using [3H]acetyl histone as substrate measured after 15 mins by liquid scintillation...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Mus musculus)
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 360nMAssay Description:Inhibition of recombinant mouse HDAC6 expressed in HEK293 cells using [3H]acetyl histone as substrate measured after 15 mins by liquid scintillation ...More data for this Ligand-Target Pair