null
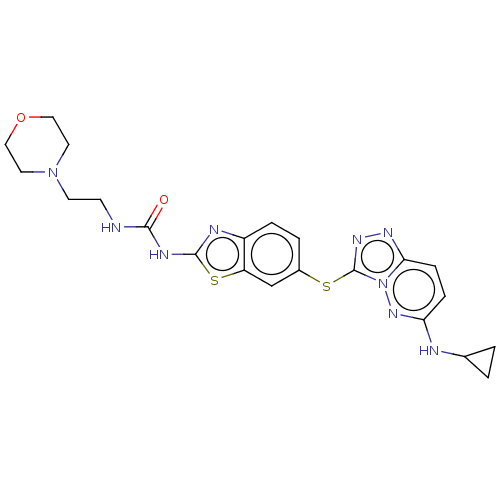
SMILES O=C(NCCN1CCOCC1)Nc1nc2ccc(Sc3nnc4ccc(NC5CC5)nn34)cc2s1
InChI Key InChIKey=MRFFMALERXPRTF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50532762
Found 6 hits for monomerid = 50532762
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of wild type phosphorylated MET (unknown origin) pre-incubated for 30 mins before biotinylated poly(glutamate-alanine-tyrosine) peptide ad...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of wild type phosphorylated MET (unknown origin) pre-incubated for 30 mins before biotinylated poly(glutamate-alanine-tyrosine) peptide ad...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant CYP3A4 co-expressed with human P450 reductase and human b5 reductase assessed as reduction in 7-Hydroxyquinoline prod...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant CYP3A4 co-expressed with human P450 reductase and human b5 reductase assessed as reduction in 7-Hydroxyquinoline prod...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Affinity DataIC50: 274nMAssay Description:Inhibition of phosphorylated MET Y1230H mutant (unknown origin) pre-incubated for 30 mins before biotinylated poly(glutamate-alanine-tyrosine) peptid...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Sanofi-Aventis Germany GmbH
Curated by ChEMBL
Affinity DataIC50: 274nMAssay Description:Inhibition of phosphorylated MET Y1230H mutant (unknown origin) pre-incubated for 30 mins before biotinylated poly(glutamate-alanine-tyrosine) peptid...More data for this Ligand-Target Pair