null
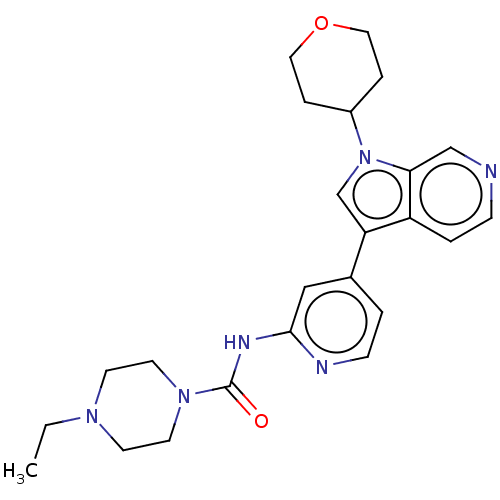
SMILES CCN1CCN(CC1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12
InChI Key InChIKey=SAZIAQSVBIWIDU-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50538099
Found 8 hits for monomerid = 50538099
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by Qpatch S8 assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 I (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 II (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)