null
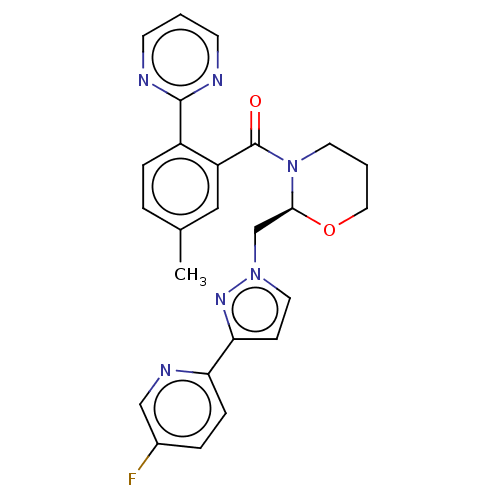
SMILES Cc1ccc(c(c1)C(=O)N1CCCO[C@H]1Cn1ccc(n1)-c1ccc(F)cn1)-c1ncccn1
InChI Key InChIKey=REIHLDGDOFIXTP-QHCPKHFHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50543839
Found 2 hits for monomerid = 50543839
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub...More data for this Ligand-Target Pair
TargetOrexin/Hypocretin receptor type 1(Homo sapiens (Human))
Taisho Pharmaceutical Co., Ltd.
Curated by ChEMBL
Taisho Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human recombinant OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub...More data for this Ligand-Target Pair