null
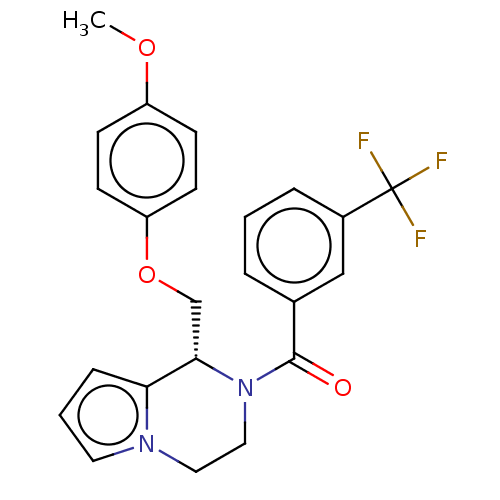
SMILES COc1ccc(OC[C@@H]2N(CCn3cccc23)C(=O)c2cccc(c2)C(F)(F)F)cc1
InChI Key InChIKey=GIZLYZGHJCGILX-NRFANRHFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50545718
Found 2 hits for monomerid = 50545718
Affinity DataEC50: 1.26E+3nMAssay Description:Positive allosteric modulation of recombinant rat GluN1a/GluN2D receptor expressed in Xenopus laevis oocytes assessed as increase in glycine/L-glutam...More data for this Ligand-Target Pair
Affinity DataEC50: 1.26E+3nMAssay Description:Positive allosteric modulation of recombinant rat GluN1a/GluN2C receptor expressed in Xenopus laevis oocytes assessed as increase in glycine/L-glutam...More data for this Ligand-Target Pair