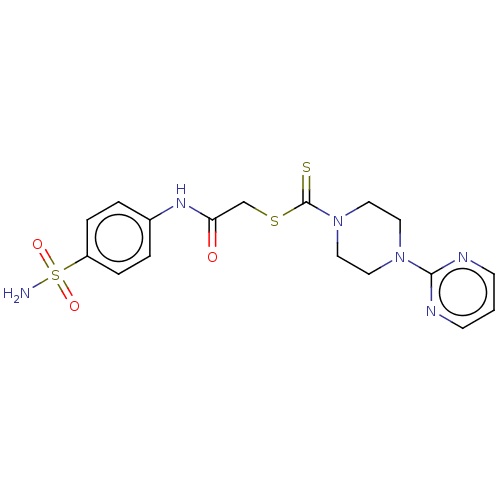
null
SMILES NS(=O)(=O)c1ccc(NC(=O)CSC(=S)N2CCN(CC2)c2ncccn2)cc1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50552008
Found 4 hits for monomerid = 50552008
Affinity DataKi: 41nMAssay Description:Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 107nMAssay Description:Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of human erythrocyte CA1 using 4-nitrophenyl acetate as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 191nMAssay Description:Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by spectrophotometryMore data for this Ligand-Target Pair