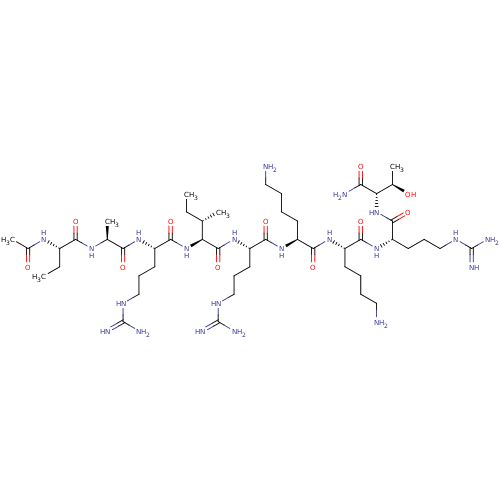
null
SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(N)=O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50552879
Found 1 hit for monomerid = 50552879
Affinity DataKi: 6nMAssay Description:Inhibition of recombinant soluble human furin using pyroGlu-Arg-Thr-Lys-Arg-methyl-coumaryl-7-amide as substrate measured after 1 hrMore data for this Ligand-Target Pair