null
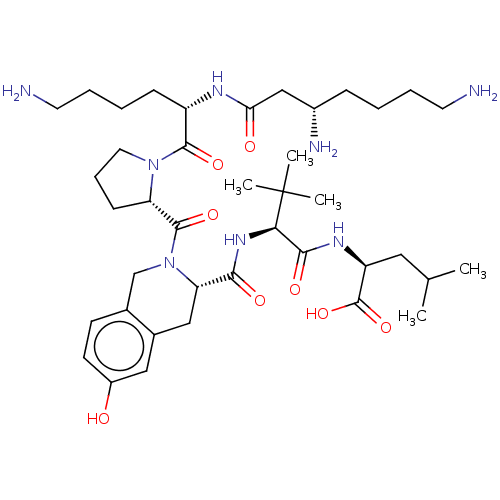
SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1Cc2cc(O)ccc2CN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C[C@@H](N)CCCCN)C(C)(C)C)C(O)=O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50553778
Found 2 hits for monomerid = 50553778
Affinity DataKi: 2.90nMAssay Description:Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter...More data for this Ligand-Target Pair
Affinity DataKi: 3.79E+3nMAssay Description:Displacement of [125I]-neurotensin from human recombinant NTS1 stably expressed in CHO-K1 cell membranes incubated for 60 mins by gamma counter metho...More data for this Ligand-Target Pair