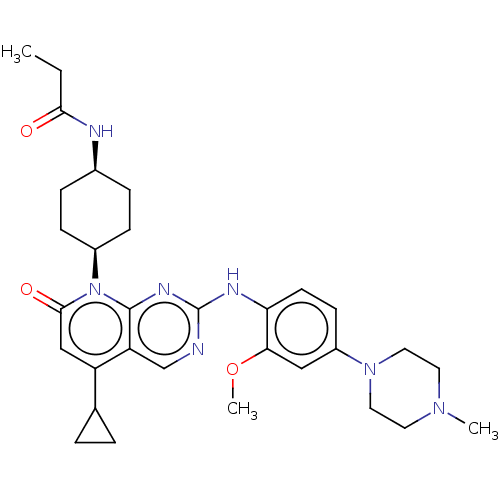
null
SMILES CCC(=O)N[C@H]1CC[C@H](CC1)n1c2nc(Nc3ccc(cc3OC)N3CCN(C)CC3)ncc2c(cc1=O)C1CC1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50569986
Found 2 hits for monomerid = 50569986
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant full-length human TTK using MBP as substrate incubated for 40 mins in presence of [gamma33P]ATP by scintillation counting b...More data for this Ligand-Target Pair
Affinity DataKd: 7.90nMAssay Description:Binding affinity to wild-type human partial length TTK (N505 to V798 residues) expressed in bacterial expression system by active site-directed compe...More data for this Ligand-Target Pair