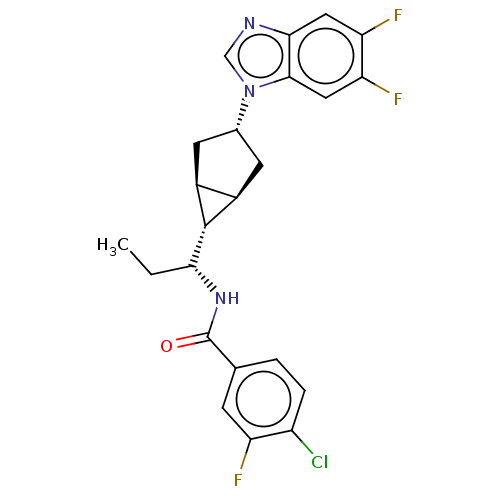
null
SMILES [H][C@]12C[C@@H](C[C@@]1([H])[C@]2([H])[C@@H](CC)NC(=O)c1ccc(Cl)c(F)c1)n1cnc2cc(F)c(F)cc12
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50578672
Found 2 hits for monomerid = 50578672
Affinity DataIC50: 2.50E+3nMAssay Description:Displacement of Tracer Red from human ERG measured after 4 hrs by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assayMore data for this Ligand-Target Pair