null
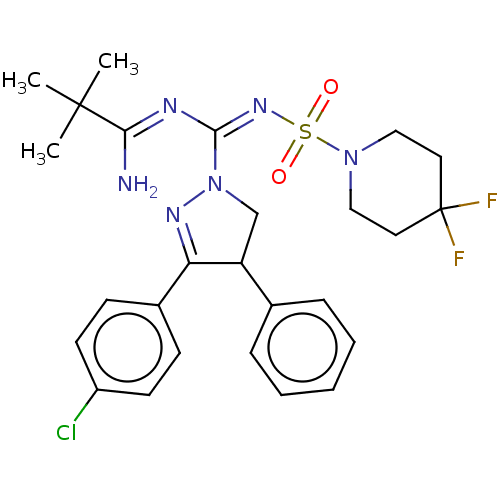
SMILES CC(C)(C)C(\N)=N\C(=N\S(=O)(=O)N1CCC(F)(F)CC1)\N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50585656
Found 4 hits for monomerid = 50585656
Affinity DataKi: 21nMAssay Description:Displacement of [3H]- CP55940 from human CB1R expressed in CHO-K1 cells membrane by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]- CP55940 from human CB2R expressed in CHO-K1 cells membrane by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human CB1R expressed in CHO-K1 cell membranes assessed as stimulation of [35S]-GTPgammaS binding by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inverse agonist activity at human CB1R expressed in CHO-K1 cell membrane assessed as increase in [35S]GTPgammaS bindingMore data for this Ligand-Target Pair